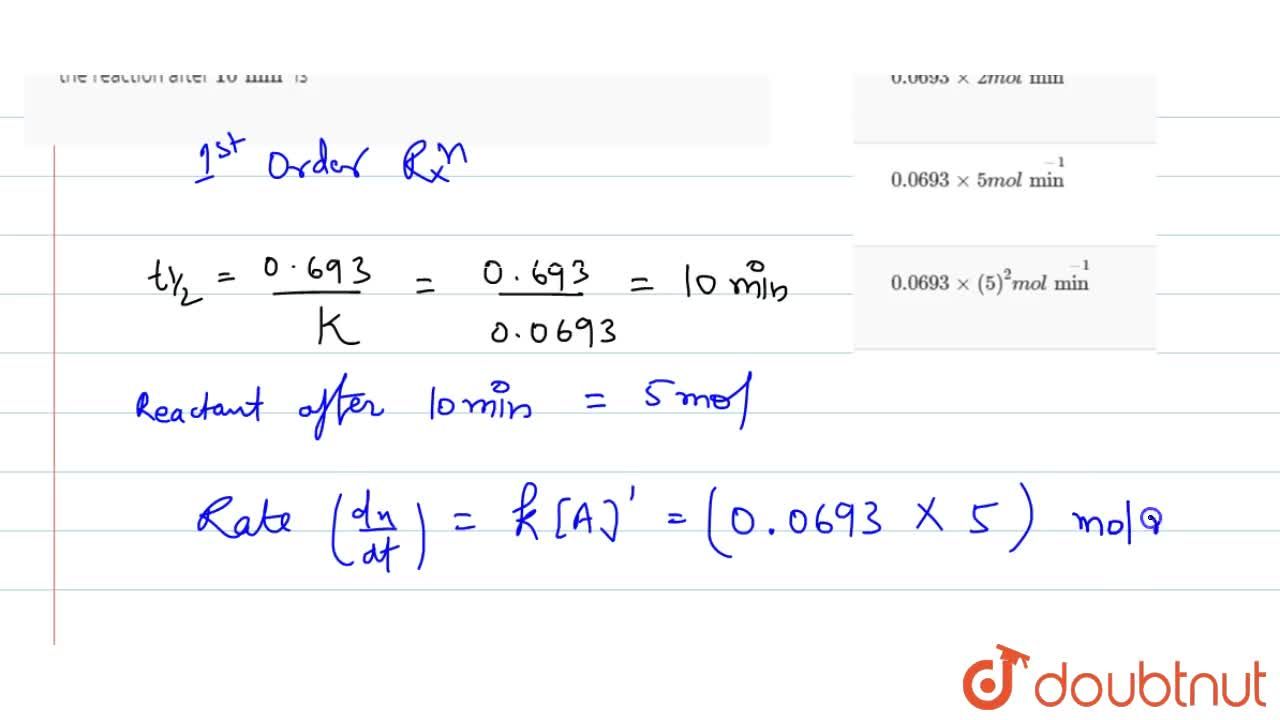
The rate constant of a reaction is 0.0693 min^(-1). Starting with 10 mol, the rate of the reaction after 10 min is
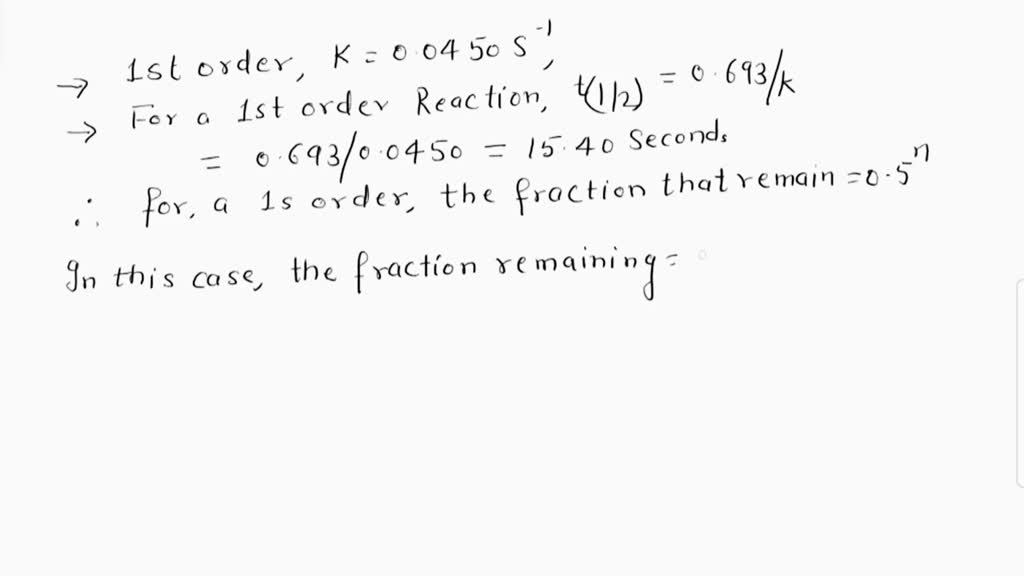
SOLVED: If a reaction is first order with a rate constant of 0.0450 s⁻¹, how much time is required for 65% of the initial quantity of reactant to be consumed?

The rate constant of a reaction is 0.69 xx 10^(-1) and the initial concentration is 0.2 "mol l"^(-1). The half-life period is

Calculate the half life of a first order reaction from their rate constants given below:(a) 200 s^-1 ; (b) 2 min^-1 ; (c) 4 year^-1 .

At a certain temperature, the rate constant of a first - order reaction is 1.40 min ^-1 . Find its half - life.

Calculate the half life of a first order reaction from their rate constants given below:(a) 200 s^-1 ; (b) 2 min^-1 ; (c) 4 year^-1 .
A first order reaction completes 50% at the end of 50 minutes. What is the value of rate constant in sec^-1? How many times will the reaction be complete at 87.5%? - Quora

For a reaction A ⟶ B + C . it was found that at the end of 10 minutes from the start the total optical rotation of the system was 50^o and

SOLVED: The potassium isotope K-40 undergoes beta decay with a half-life of 1.83*10^9 years. Find the number of beta decays that occur per second in 1.0g of pure K-40.

In the following gaseous phase first order reaction A(g) → 2 B(g) + C(g) initial pressure was found to be 400 mm of Hg and it changed to 1000 mm of Hg

For a reaction A ⟶ B + C . it was found that at the end of 10 minutes from the start the total optical rotation of the system was 50^o and

A first order reaction is found to have a rate constant k= 5.5 xx 10^(-14)s^(-1). Find half-life of the reaction.

If the half life period for a first order reaction is 69*3 seconds, what is the value of its rate constant ?

Half life of a first order reaction is 2.1xx10^(12)s. Calculate the rate constant of the reactio... - YouTube
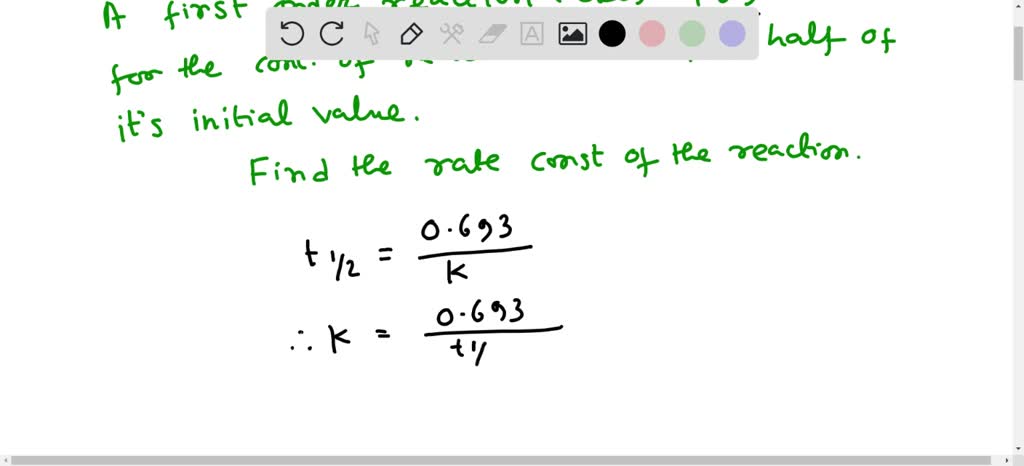
SOLVED: What is the rate constant of a first-order reaction that takes 456 seconds for the reactant concentration to drop to half of its initial value?
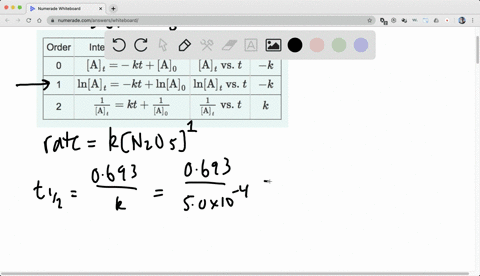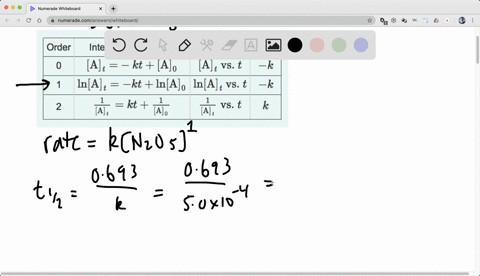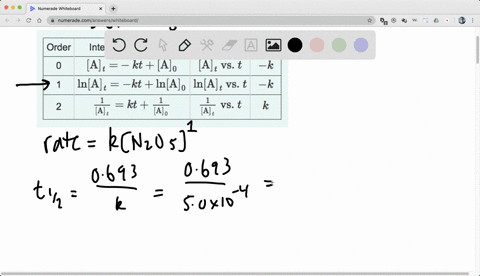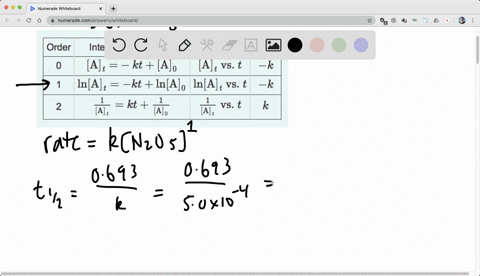
Chapter 15, Principles of Reactivity: Chemical Kinetics Video Solutions, Chemistry and Chemical Reactivity | Numerade
✓ Solved: The rate constant for a certain radioactive nuclide is 1.0 × 10^-3 h^-1 .What is the half-life...